Chlorine Dioxide | No.1 Choice for water disinfection
|
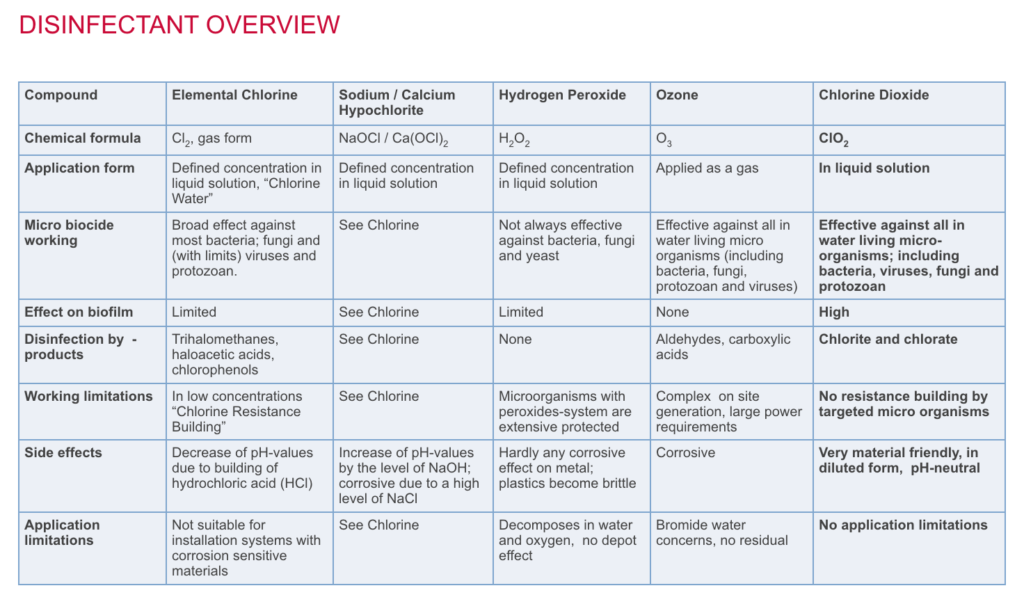
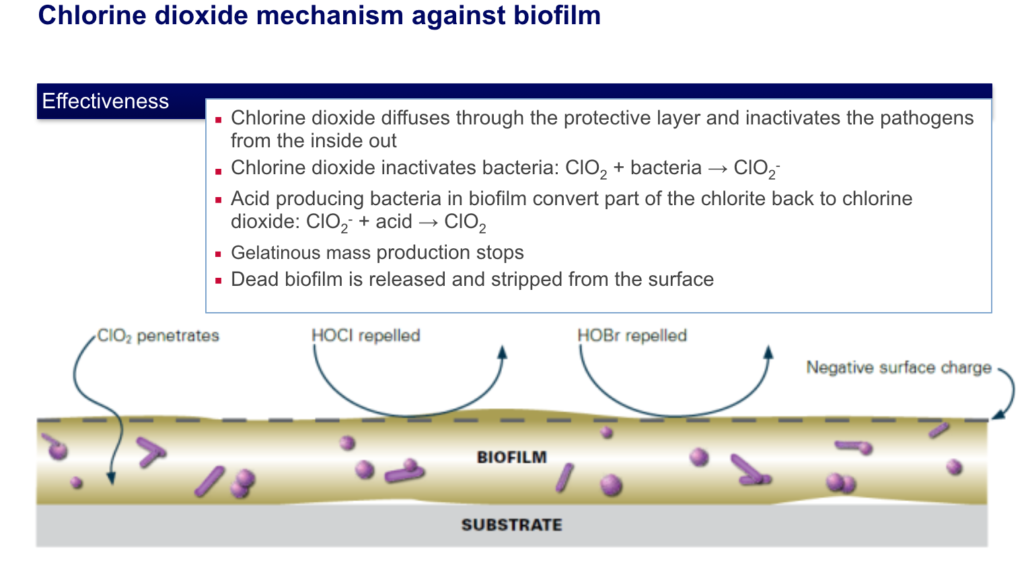
Chlorine Dioxide is effective against all types of fungi, algae, and bacteria. Investigations have shown chlorine dioxide to be more sporicidal than chlorine. Even at low concentrations between 0.3 and 0.5ppm, a high level of disinfection can be achieved.
In water, chlorine dioxide does not chlorinate organic compounds such as naturally occurring phenolics. Unlike chlorine, chlorine dioxide does not produce tri-halo methane type chemicals or absorbable organic halogens. Both of these type chemicals are seen as highly undesirable in the environment. Chlorine dioxide retains its activity at pH levels in excess of 9.0 whereas chlorine shows a significant loss of activity at pH levels in excess of 7.5. |
|
Chlorine dioxide residuals remain present for longer periods than chlorine in the treated water. This means that chlorine dioxide treated water retains microbiological quality for longer which will have the following benefits on site:
|
Treated water can be piped for longer distances within the site without compromising microbiological quality.
|
Treated water supplied to water handling plants, hygiene washing plants and evaporating cooling towers is of a considerably higher microbiological quality compared to chlorine-based biocides.
The investigation has shown that subsequent microbiological control of water used in such equipment is easily achieved, often with some economies in further biocide use. |
Chlorine dioxide timeline
- 1811 first discovered by Sir Humphrey Davey
- 1944 First commercial application. Used as a biocide/taste and odour control agent in domestic water at Niagara Falls in the USA.
- 1977 Three thousand municipal water systems achieving biological control using Chlorine Dioxide.
- 1980’s Chlorine Dioxide gradually replacing Chlorine in many industries.
Industrial water treatment – used as a biocide and as an odour control agent.
Food processing – used as a sanitiser.
- 1990’s Increasingly used for the secondary disinfection of potable water.
- 2005 – New generation equipment and control technology introduce ClO2 as a practical alternative to many industrial applications.
ClO2 Factfile
- What is Chlorine Dioxide? Chlorine Dioxide is a small, volatile and very strong molecule consisting of 1 Chlorine atom and 2 oxygen atoms. Abbreviated to ClO2, Chlorine Dioxide exists as a free radical in dilute solutions
- Molecular weight of 67.45.
- It is a gas at normal temperatures and pressures.
- Melting point of -59oC.
- Boiling point of 11oC.
- Yellowish/green colour and has an odour similar to that of Chlorine.
- Denser than air and is water-soluble at standard temperatures and pressures up to 2500ppm.
- Explosive in air at concentrations >10%. It is therefore normally generated in-situ within an aqueous solution at <0.2%
- It will decompose in the presence of UV, high temperatures >70oC, and high alkalinity (>pH12).
uses and benefits of using chlorine dioxide
- Powerful Disinfection in Water Treatment
- Chlorine dioxide is a disinfectant.
- When added to drinking water, it helps destroy bacteria, viruses and some types of parasites that can make people sick, such as Cryptosporidium parvum and Giardia lamblia.
- The Environmental Protection Agency (EPA) regulates the maximum concentration of chlorine dioxide in drinking water to be no greater than 0.8 parts per million (ppm).
Chlorine Dioxide can be applied to the following:
Chlorine dioxide chemistry is used in a wide variety of industrial, oil and gas, food and municipal applications:
- Vegetable processing
- Food and Beverage Production – Chlorine dioxide can be used as an antimicrobial agent in water used in poultry processing and to wash fruits and vegetables.
- Poultry processing
- Brewing
- Borehole water
- Potable water
- Legionella control
- Paper Processing – Chlorine dioxide is used to chemically process wood pulp for paper manufacturing
- CIP rinse waters
- Other water disinfection, cool water, process water, hot and cold water services.